After more than 150 years, the sensory secrets of penis and clitoris have been unlocked
Training of the oestrus cycle in female mice using the first and second cycles of mating and sex sessions in the laboratory with experienced males
Only experienced males were used in the testing of female mice. The same group of female controls and mutants were hormone-primed (as described above) in the first two rounds of the mating test, while, in the third round, they were in a natural oestrus cycle. The first two rounds were conducted 2 weeks apart, and the third trial was conducted 4 weeks after the second trial to allow for the recovery of oestrus cycle. The oestrus cycle was monitored using the vaginal cytology method according to previously established protocols71,72. Vaginal samples were stained in a lab with crystal violet to determine the oestrus stage. Female mice were only used for sex within 3 h of vaginal lavage. The female mice that were treated were able to engage in the full test in the first and second rounds, in order to get sexual experience for the cohort. In the third test for the same groups of animals but under their natural oestrus cycle, females were separated from the males 10 min after the start of intromission to avoid pregnancy. If no intromission occurred, the mating session was terminated at 30 min.
In order to kill patifen, it was dissolved in 100% ethanol, diluting with 1:2 in seed oil and vacuum-centrifuged for 30 min. The population was densely labelled with an injection of a small amount of a drug in the abdominal area at P5 and also with a tiny amount of a drug in the brain at P5. In some cases the pregnant mother was given a pill called tamoxifen to label the populations that were the same. The Ret+ population was densely labelled with 3 mg of tamoxifen delivered to the pregnant mother through oral gavage at E11.5 or E12.5 and sparsely with 0.5 mg at E12.5.
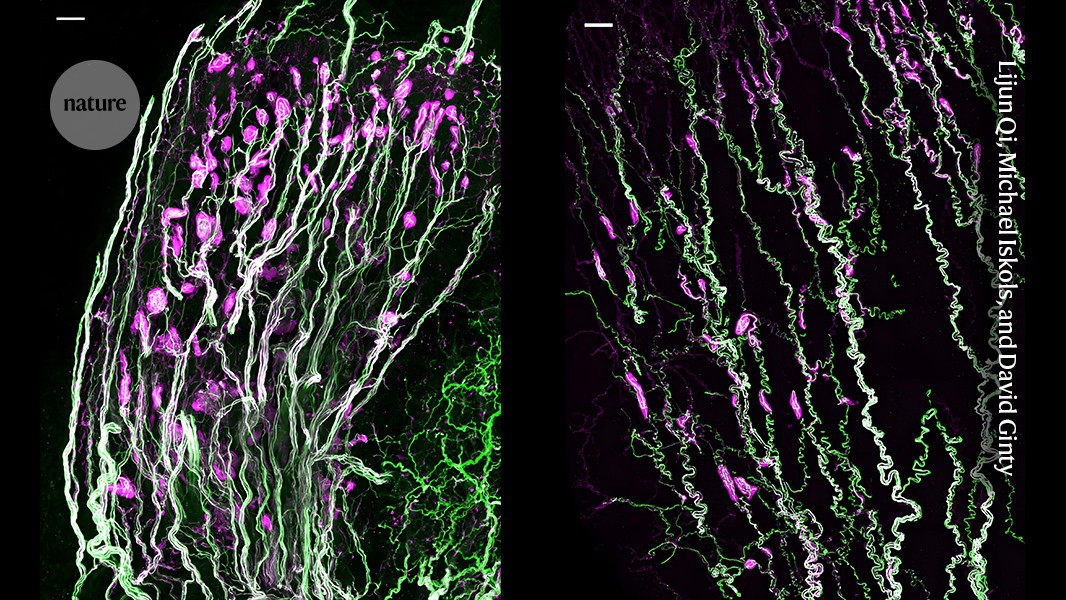
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Immunohistochemistry of clitoris dissection of a female penis and a paw using whole-mount AP staining
The clitoris dissection used different methods to remove the cells of the female genitals and allow for the spreading of the clitoris. The superficial layer between the os penis and the internal mesenchyme was removed to make way for a visualization of the penile innervation. While not included in our primary analysis, the internal prepuce of the penis was retained for whole-mount AP staining. The prostatic urethra was not included in the analysis as it was removed and a cut made to allow flattening of the tissue. The paw’s entire surface was removed from its underlying tissue for staining. The hairs on the back and around the genitalia were removed using Nair. The whole spine was removed, and the overlying dura was removed, in order to attach the dorsal column nucleus.
Immunohistochemistry of thick sections was performed using previously described protocols for whole-mount tissue56. Sections were washed in 0.3% PBST five times for 1 h and incubated in primary antibody in blocking solution (5% normal donkey serum, 75% PBST, 20% DMSO) at room temperature for 3–5 days under gentle agitation. After five 1 h washes with 0.3% PBST, secondary antibodies in blocking solution were added for 2–4 days at room temperature. The sections were then washed twice in 0.3% PBST for 1 h, stained with DAPI (1 µg ml−1, 5 min) to visualize nuclei, further washed three times in 0.3% PBST for 1 h, and dehydrated in serial methanol concentrations (50%, 75%, 100%, 1 h each) and then overnight in 100% methanol. The specimen was removed from the BABB and then imaged on a confocal microscope or stereoscope submerged in water. The tissue was kept at a temperature of 4C.
The primary antibodies used in this study were as follows: chicken anti-GFP (Aves Labs, GFP-1020, 1:500), goat anti-GFP (US Biological, G8965-01E, 1:500), goat anti-mCherry (CedarLane, AB0040-200, 1:500), rabbit anti-CGRP (Immunostar, 24112, 1:500), chicken anti-NF200 (Aves Labs, NFH, 1:200), rabbit anti-NF200 (Sigma-Aldrich, N4142-.2ML, 1:500), rabbit anti-S100 (ProteinTech, 15146-1-AP, 1:200–1:500), rat anti-TROMA-1 (DSHB, AB_531826, 1:200), goat anti-CD31 (R&D Systems, AF3628, 1:500), guinea pig anti-Flag (1:500)31 and IB4 (Alexa 647 conjugated) (Invitrogen, I32450, 1:500).
To find the total number of corpuscles per animal, all of the 200 m sections from a given clitoris or penis sample was collected and stained with S 100 and NF200 to determine their total number. The sections were imaged under a confocal microscope. The location of each corpuscle is preserved in an ROI file as part of the count. The borders of imaged tissue were manually outlined and saved. Each section’s volume was computed by multiplying the area of the outlined region by the height of the z stack, allowing for calculation of corpuscle density. To generate distribution heat maps, the ROI files containing the location of each corpuscle and the tissue outline were imported into MATLAB. The distribution of corpuscles was binned in a grid, and a Gaussian filter was used to smooth the density distribution.
Complex Krause corpuscles were defined as Krause corpuscles containing tightly coiled axons. Complicated and short-lived forms were seen in some Complex Krause Jacacles, as well as long and convoluted profiles. The Simple Krause corpuscles contained 1 or 2 linear axons. All of the simple forms have the same shape, which makes them 888-609- 888-609- 888-609- 888-609- 888-609-
Dense MRGPRB4+ tissue labelled by AAV-FLEX-PLAP64 in PBS and B3 buffer for cryogenic enzymatic reactions
The samples were smicated in cacodylate buffer with 1% osmium tetroxide. The sections were washed with double distilled H2O and stained with a solution of 0.05 M sodium maleate (pH 5) and 1% uranyl acetate. The sections were dehydrated after washing with double-distilled H2O. The sections were then put to sleep at 4 C overnight and then injected with a mix of epoxy resins and propylene oxide. The sections were embedded in an epoxy resin mix and cured at 60 °C for 48–72 h. Ultrathin (approximately 60 nm) sections were generated and imaged on the JEOL 1200EX transmission electron microscope at 80 kV accelerating voltage. The images were smaller than the original ones.
TrkB+ and Ret+ afferents were sparsely labelled using the Brn3acKOAP placental AP (PLAP) reporter mouse55, as described above, to enable visualization of single nerve terminals in genital and glabrous tissue. The C-LTSRs were densely labelled using Th2A-creER;Brn3acKOAP animals. The child was administered at 3 weeks old. sympathetic fibers are not labelled in animals because they don’t express BRN3A. The MRGPRB4+ afferents were labelled using intrathecal injections of AAV-FLEX-PLAP64 (5 μl, titre 1.2 × 1013 genome copies per ml) into 4-week-old Mrgprb4cre mice58. All of the mice had to be perfused after a few weeks.
The post-fixed and dissected tissue was incubated in PBS at 68 °C for 2 h to inactivate endogenous AP, then rinsed three times for 5 min in B3 buffer (0.1 M Tris pH 9.5, 0.1 M NaCl, 50 mM MgCl2, 0.1% Tween-20) at room temperature. For the PLAP enzymatic reaction, tissue samples were incubated at room temperature overnight in BCIP and NBT in B3 buffer (3.4 µl of each per 1 ml of B3 buffer). Stained tissue was then pinned flat in a dish, dehydrated in 4% PBS and then covered with 50%, 75%, 100%, and 100% Ethanol for an entire day. The tissue was removed and imaged in BABB using a stereoscope.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Corpuscles as genital vibrotactile sensors for sexual behaviours: Reconstruction and filling of individual arborizations
For analysis, individual arborizations were imaged. The terminal area was measured in ImageJ by drawing a tight polygon around the terminals of a given axon, and the number of corpuscles innervated by each fibre was counted manually. It is possible that the corpuscles of a given afferent occupy a larger volume in the z dimension than is measured in this manner. The thin and linear terminals of simple corpuscles were considered to be better than the wider and deeper terminals of complex corpuscles. The reconstruction and filling of fibres was done using an imageJ SNT Plugins.
Young adult mice were anaesthetized with continuous inhalation of 2% isoflurane from a precision vapourizer for the duration of the procedure (5–10 min). The injection was done with a glass pipette. The glass pipette was connected to an aspirator tube assembly (Sigma-Aldrich, A5177-5EA), which was connected to a syringe to control the air pressure.
For injection of mid-line hairy skin, hairs near the genital protrusion in either male or female were removed by Nair treatment and subsequently cleaning using 70% ethanol. The glass pipette was put into the superficial hairy skin by means of a pair of blunt forceps. Immediately after the injection, the fast green dye was visible within the hairy skin region.
A total volume of 2 μl was injected into either the male or female target region, for injection of either CTB or AAV. For CTB injections, 4–8 week old animals were used, and the injected animals were perfused in 3–4 days. The injected animals were perfused for 3 weeks after being administered AAV2 retro-hSyn-FlpO.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Measurement of axial levels of the lumbosacral spinal cord using an open-source microcontroller with Intan Technologies ImageJ macro code
Owing to the characteristic decrease in the size of the dorsal column from the lumbar enlargement to more caudal regions of the spinal cord, the axial levels of the lumbosacral spinal cord sections were determined by the ratio of the depth of dorsal column (Ld) to that of the central canal (Lc). Ld was calculated as the length from the midpoint of dorsal surface of spinal cord to the ventral border of dorsal column, whereas Lc was calculated as the distance between the midpoint of dorsal surface of spinal cord and the central canal. Ratios of Ld/Lc from the images in the reference atlas of the mouse spinal cord (https://mousespinal.brain-map.org/imageseries/showref.html) were calculated and used as the reference to identify axial levels of the lumbosacral sections.
The platform was then moved to the MEA recording setup. A 32-channel silicon probe (Cambridge NeuroTech, ASSY-37 H6b) was inserted into either the left or right L6 DRG. The signals were amplified and recorded using an Intan Technologies RHD2132 amplifier chip and RHD USB Interface Board. Open-source software was used to control data acquisition.
motion correction was performed using a custom-written ImageJ macro code that utilized the moco and Unsharp mask filter, and then regions of interest were manually selected. Cells showing a baseline signal or calcium response were identified and aligned across the videos. The intensity measurements generated by ImageJ were then processed for further analysis using MATLAB. The baseline activity was used for the computation of F/F. The mechanical threshold was set based on the first calcium spikes aligning with the step. To determine the mechanical thresholds for vibrations at varying frequencies, the s.d. of the baseline activity was calculated and the threshold for the response was defined as 5 × s.d. above the baseline. Next, the recorded force at that timepoint was defined and averaged across trials with the same frequency. To differentiate between clitoris-innervating neurons and neurons innervating hairy skin, only neurons that responded to electrical stimulation (5 × s.d. above the baseline) were included for further analysis.
Automated clustering of action potentials in genitalia and the latency of optotagged neuronal conduction velocities
JRCLUST was used to automatically sort action potentials into clusters, which were then manually refined and classified as single or multi units (https://github.com/JaneliaSciComp/JRCLUST).
To calculate the conduction velocity of optotagged neurons, the latency was determined by subtracting the time of each spike from the middle point of each light pulse. The latencies were for four optotagged rhyolite afferents. The length of axonal projections from L6 DRG to the genitalia in adult mice was estimated to be 5 cm. The four optotagged neurosciences have the same conduction velocities of 6.4 m s1, 11.4, and 3.7.
To determine the mechanical thresholds for vibration stimuli across different vibration frequencies, the time of the first spike in response to the vibration in each trial was identified. The corresponding recorded force at that timepoint was defined as the mechanical threshold for that trial. Thresholds of the trials at each vibration frequency were then averaged.
Source: Krause corpuscles are genital vibrotactile sensors for sexual behaviours
Tactile prepopulse inhibition of a mouse model animal using an upright epifluorescence microscope: baseline measurement and startle response
The surgery procedure for male mice was the same as for the MEA recordings above. After the L6 DRG was revealed, the platform was moved to an upright epifluorescence microscope with an 10 air objective. The light source was a 470 nm LED (M470L5, Thorlabs) with an LED driver (LEDD1B, Thorlabs), and a CMOS camera (CS505MU1, Thorlabs) was triggered at 10 frames per second with 50 ms exposure time using ThorCam software (v.3.7.0). The stimuli were synchronized with the camera using a data-acquisition board.
For thermal stimulation of the penis, a water reservoir device, inspired by a previously described method66, was used. The device was connected to water baths at various temperatures, including room temperature, ice water, and hot water. The penis was in the water. Then, water of different temperatures was pumped into the reservoir at a controlled speed, and room-temperature water was used during the baseline measurements. The water was directed to a section of the body of water. The temperature was measured from a thermocouple microprobe as well as a monitor (BAT-12).
The sticky-tape assay as a measure of hairy skin sensitivity was performed using a previously described protocol67. A piece of laboratory tape was secured to the back of the animal. Then, the animal was introduced into a 15 cm by 15 cm Plexiglass enclosure and video-monitored for a period of 5 min. The attempts to remove the tape by the animal were quantified using a software.
The tactile prepulse inhibition (PPI) experiments were conducted according to a previously described procedure68. The mouse was positioned inside a plastic cylinder with 3.8 cm diameter. The container was then secured in a soundproof chamber (SR-LAB Startle Response System). Before an acoustic startle stimulus (125 dB, 20 ms), a 0.9 psi air puff (50 ms) as a prepulse was delivered to the back of the animal at various interstimulus intervals (50, 100, 250, 500 and 1,000 ms). The animal’s startle response was recorded using an appliance that measures movement in the air. PPI was calculated as %PPI = [1 − (startle response from pulse following prepulse/startle response from pulse alone)] × 100.
After securing the animal, balloon and stimulator, it was possible to place the stimulator at the top of the clitoris. When a stable baseline was established, a 50 hertz wave could be delivered with a 1 s duration followed by a 0.2 s interstimulus interval. Each session consisted of 10 such epochs, lasting a total of 15 s. The stimuli were delivered using a program. More than three Vibration sessions were conducted for each animal. Each mouse was then anaesthetized with 1.5% isoflurane, anaesthesia was verified by toe pinch and additional vibration sessions were conducted. The start of the vibration session was manually annotated in the AcqKnowledge software. The movement of the mouse was monitored throughout the experiment and all movement artifacts were marked accordingly in the software.
After a spine injury, reflexes were tested at 6 or 24 h. The animals hind limbs were secured with Scotch tape on the platform after they were restrained in a round chamber. Two cameras were positioned to look at the genital region of the mouse from different angles. The mouse’s glans penis was externalized by applying pressure to the side of the genital region. Reflexes might have occurred during or right after the externalization due to the force applied in this process, but this effect would end within 1 min of externalization.
The sexual reflexes were stimulated two minutes after the glans penis was taken out of the animals. The optic fibre (diameter, 1,000 µm; 0.39 NA; M35L02, Thorlabs) connected to a fibre-coupled LED (M470F4, Thorlabs) was directed towards the glans penis. The stimulation sessions lasted 20 s, with 10 Hz and 2 ms pulses used for animals expressing ReaChR, and 20 Hz and 1 ms pulses used for animals expressing CatCh. The maximum light intensity was 10 mW.
Female mice were treated with 17-estradiol benzoate two days before the test. to facilitate access to the vaginal opening. The day before testing, mice were spinal transected at the T9/T10 level, using methods described above. On the day of the test, the awake, paralysed animals were gently restrained in a supine position with all four limbs taped on a platform, to reduce motion artifacts during the pressure measurements. A balloon was placed into the vagina to secure it, and a pipe was taped to the platform. The assembly was connected to a pressure transducer (BIOPAC system, TSD104A) through saline-filled tubing. The output of the transducer was amplified at a 2000hz sampling rate with the help of abioPAC mp150 system and AcqKnowledge software.
The videos of mating behaviours were manually scored using BORIS software69 by an experimenter who was blinded to the genotype of the animals. Sniffer, mount, and ejaculation were the four main male behavioural behaviors that were scored. Mounting refers to the males’ rapid pursuit of females, followed by grasping their rear, and often followed by short probing without gaining access to the female genitalia. It indicates the male is successful in penetrating the vagina with long rhythmic thrusting. The mounting and intromission periods do not overlap with the scoring methods used; mounting is considered to be the pursuit and adjustment period before the intromission period. The intromission bouts lasting less than 2 s were excluded from the analysis due to the lack of rhythmicity of movements and the possibility that penetration did not fully occur. Female mice were scored on their combative and darting behaviours during the female mating behavior assessment trials. The combative and dart behavior are related to instances in which the female confronts or fights with the male.
The data was analysed using a number of programs. When length is allowed, the samples and statistical tests used in individual experiments are included in the figure legends. In Fig. 2c, n = 10 sections from 3 control females, 10 sections from 3 TrkBcKO females, 7 sections from 2 control males and 20 sections from four TrkBcKO males. In the figure. Thirty-eight axons, from 5 males, 16 Ret+ axons from 3 males, 26 axons from 4 females, and 26 axons from 3 females.
There is very little research into how the genitals work and how they are involved in sex, because the topic is considered taboo. “It’s been hard to get people to work on this because some people have a hard time talking about it,” says David Ginty, a sensory neurobiologist at Harvard Medical School in Boston, Massachusetts, who led the team that conducted the latest research. “But I don’t, because the biology is so interesting.”
Sensory biologists have long wanted to study these balls. But activating and tracking specific neurons was nearly impossible until advanced molecular techniques emerged in the past 20 years.
Although most sensory neurons are developed before birth, the researchers found that Krause corpuscles didn’t develop until the mice were around 4–6 weeks old — just before the animals reached sexual maturity. Ginty says the team is studying the hormones in the oestrus cycle of the mouse to see if they affect the function of the corpuscles.
Alexander Chesler, a sensory biologist at the National Center for Complementary and Integrative Health in Bethesda, Maryland, says that the study complements a paper his group published last year2 showing that a touch-sensitive protein in the genitals is necessary for successful mating. Sex is a fundamental area of biology and one of the main drivers of evolution. He hopes that this research will lead to treatments for a lot of conditions, such as sexual problems and vaginal pain.