The Survival Analysis of Diet Groups and Genome-Scan Models. I. Covariate Analysis and Genetic Mapping Analysis of Lifetime Outcomes
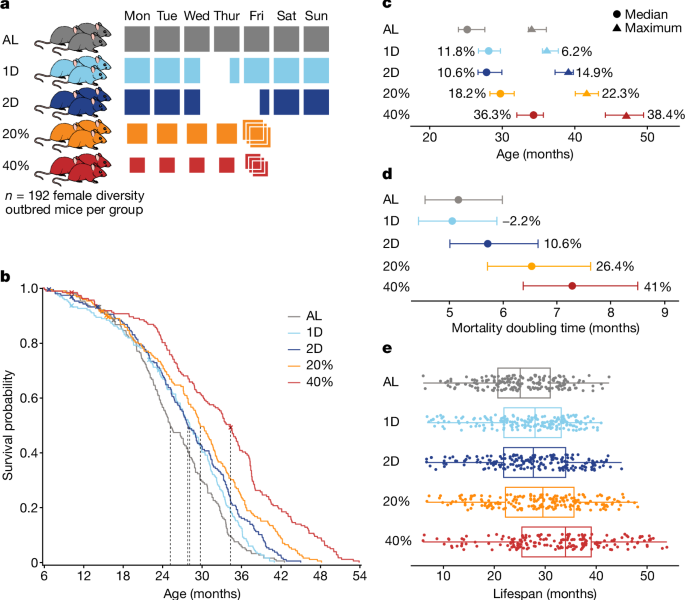
The survival analysis compares lifespan outcomes for the five study groups. We plotted Kaplan–Meier survival curves, used log rank tests and all pairwise comparisons between diet groups to find the equality of survival distributions. P values are reported without testing adjustments, and we considered the comparison to be significant. if P < 0.01. We estimated the median and maximum lifespan (90% survival) by diet group with 95% confidence intervals and percentage change relative to the AL group30. The doubling times were estimated from a log-linear hazard model that had a percentage change compared to the AL group.
The results drive home the nature of the body’s response to restriction of calories. James Nelson is a biogerontologist with the University of Texas Health Science Center in San Antonio.
The study was published today in Nature by Churchill and his co-authors, including scientists at Calico Life Sciences in South San Francisco, California, the anti-ageing focused biotech company that funded the study.
We performed a regression analysis on lifespan with traits at each time point after adjusting for effects of diet and body weight. We fit linear models.
Genetic mapping analysis of all continuous-valued traits including lifespan was performed using the R package qtl263. diet and body weight were two variables that were co-variables in the genome-scan models. Founder haplotype effects were estimated treating 8-state genotypes as random effects. Est.herit was used to estimate typic heritability using a linear mixed model. Confidence intervals were obtained by parametric bootstrap. Significance thresholds for QTL mapping were estimated from 1,000 permutations of the trait and covariate data; significant QTL exceed the genome-wide multiple-test adjusted 0.05 threshold (7.5) and suggestive QTL exceed the unadjusted 0.05 threshold (6.0)64.
We obtained a correlation by re computing a sparse network with reduced representations of 23 features. To obtain the relative importance of different effects of DR on lifespan, we performed covariance path decomposition48 of the covariance between diet and lifespan using this reduced representation graphical model. The absolute values of path scores were used to rank the relative importance of each path and normalized to sum to 1 to estimate the fraction of the covariance between DR and lifespan explained. To obtain network visualizations, the position of nodes and orientation of edges were determined by computing max-flow through the path network. We define the path network as the graph formed by taking the partial correlation network but reweighting edges to be the sum of all (absolute) path scores of paths that contain that edge.
To cluster the phenotypes, we constructed a weighted k-nearest neighbours graph using the absolute partial correlation from above as similarity weights (that is, we retained the k-largest weights per node) using k = 10. The resulting k-nearest neighbours graph was clustered using spectral clustering to obtain 20 clusters, which were labelled by hand. Diet and lifespan were broken out into their own univariate clusters and the body weight cluster was split into lean tissue mass and adiposity (fat mass/total mass) to account for body composition effects known from the literature. This resulted in a summarized representation of 23 clusters (diet, lifespan and 21 groups of traits, one per cluster plus FTM).
where BW6 is the last preintervention body weight, and BWtest is the body weight at time of testing. For body composition and change-in-body weight traits, we did not include the body weight terms. All continuous variables were rank normal scores (RZ) transformed before model fitting. We performed likelihood ratio tests for the diet and body weight adjusted association (M2 versus M1) and for the diet × trait interaction (M3 versus M2). We applied FDR adjustment to each test according to trait and time point. Traits were categorized and significant (FDR < 0.01) results were tabulated as above.
We enrolled 960 female DO mice in 12 waves, corresponding to birth cohorts from generations 22 to 24 and 26 to 28 with 80 first-parity and 80 second-parity animals from each generation born around 3 weeks apart. There were no more than one mouse per litter in the study. The first cohort entered the study in March 2016 and the study was fully populated in November 2017. This schedule was designed to make efficient use of our phenotyping capacity and minimize the potential for seasonal confounding. The sample size was chosen because it could detect 10% change in mean lifespan between intervention groups with allowed loss of animals due to non-age related events. We used female mice due to concerns about male aggression. Mice were assigned to housing groups of eight animals in large-format wean boxes with positive pressure ventilation and incoming air temperature of 24.4 to 25.6 °C. Environmental enrichments were provided including nestlets, biotubes and gnawing blocks. One of the five interventions was randomized to the mice. Different feeding requirements in different intervention groups made it impossible to blind. The analysis shows that 960 mice were included in the study and 937 of them were alive at 6 months of age. All of the procedures used in the study were reviewed and approved.
Research staff regularly evaluated mice for prespecified clinical symptomology: palpable hypothermia, responsiveness to stimuli, ability to eat or drink, dermatitis, tumours, abdominal distention, mobility, eye conditions (such as corneal ulcers), malocclusion, trauma and wounds of aggression. veterinary staff were contacted if the mice met criteria in any of the categories If the clinical team determined a mouse to be palpably hypothermic and unresponsive, unable to eat or drink, and/or met protocol criteria for severe dermatitis, tumours and/or fight wounds, pre-emptive euthanasia was performed to prevent suffering; otherwise, the veterinary staff provided treatment. Both mice euthanized or found dead were represented as deaths in the survival curves. Mice euthanized due to injuries unrelated to imminent death were treated as censored (we recorded a total of 13 censoring events).
Free-wheel-running data were collected at around 44, 96 and 144 weeks of age. Mice were individually housed for a minimum of 36 h in a special cage suited to house the Med Associate low profile running wheel with a wireless transmitter. The food hopper was removed to allow for seamless movement of the wheel, and food was placed onto the cage floor. The 15.5-cm-diameter plastic wheel sits at an angle on an electronic base, which tracks the revolutions. The battery-powered base allows for continuous monitoring, whereby data is transmitted in 30s intervals to a local computer.
A piece of blotting paper was cut to fit into standard duplex cage dimensions. The shaving was removed from the cage, and the paper taped to the bottom of it. The water was removed to prevent it from pooling onto the blotting paper, which was provided with food. Mice were individually housed in a prepared cage for 4 h. At the end of the trial, the mice were returned to their original housing units, and papers were removed and dried for 2–4 h, before being individually bagged. Papers were shipped to Beth Israel Deaconess Medical Center, where they were scanned with ultraviolet light to image and quantify the void spots.
We used the Ugo–Basile rotarod, which has five lanes evenly spaced along a motorized horizontal rotating rod, allowing for up to five mice to be tested simultaneously. The platform below is used to record thelatency of each mouse to fall. The mice were placed on the rod at 4 rpm, then gradually increased to a maximum of 40 rpm, over 300 s. We reported the mean latency (time to fall) and the slope of latencies across trials, as well as the number of trials with no falls and number of trials with immediate falls. If a mouse did not cooperate, trials were recorded as missing.
Startle response was measured in rodents using automated startle chambers, in which a mouse was placed in a clear, acrylic tube attached to a highly sensitive platform that is calibrated to track their startle reflex while being exposed to a series of stimuli at varying decibels and times. The mice were exposed to white noise from a speaker for a short time, which was followed by a series of randomized, computer-generated stimuli that ranged in volume from 70 to 120 decibels. The test takes 30 minutes.
Measurement of blood flow cytometry in mice using pulse-Dopamine sonography with VEVO 770/3100 at 30 and 40 MHz
Ultrasonography was performed using the VEVO 770/3100 system with probes for 30 and 40 MHz. Blood flow rates and volumes can be measured with the aid ofechocardiography, in which pulse-Dopamine sonography is applied through the probe.
At the flow cytometry blood collections at 16, 62 and 114 weeks, mice were fasted for 4 h and glucose was measured using the OneTouch Ultra2 glucose meter from LifeScan along with OneTouch Ultra test strips. At each of the CBC blood collections at 24, 71 and 122 weeks, non-fasted glucose was measured using the glucose meter.
The immune cell subclasses of the mice were generally assigned to either naive or non-naive by the presence of CD62L and CD44, as shown in Supplementary Table 7. NKG2D-positive cells were enumerated and may represent memory T cells that accumulate after immune responses60. Owing to limitations in flow cytometry markers that identify NK cells and their subsets in the mouse strains contributing to the outbred DO mouse line, NK cells were defined as non-T non-B lymphocytes expressing NKG2D. Within this population, CD11c and CD11b were used to generally define maturation subsets. CD11b expression marks more mature NK cells and CD11c is reduced on the least-mature NK subset61.
Peripheral blood samples were analysed by flow cytometry to determine the frequency of major circulating immune cell subsets. At 5 months, 16 and 24 months of age, analysis was performed. These are the times when there was 11 and 19 months of intervention. The red blood cells in the samples were washed and lysed. Cells were resuspended in a 25 l buffer with a small amount of alcohol. Antibodies were added and put into incubated for 30 min at 4 C. Labelled cells were washed and DAPI was added before analysis on the LSRII (BD Bioscience) system. The cocktail contained CD11c FITC, N41; CD3e PE-CF594; and NKG2D (CD 314) PE.
Source: Dietary restriction impacts health and lifespan of genetically diverse mice
The LUNAr PIXImus II densitometer for the study of age-related changes in skeletal and lean tissue composition in female DO mice
We used the LUNAr PIXImus II densitometer to collect body composition and bone density. A tray of food and anaesthesia were placed onto the PIXImus exposure platform. 4 min is how long the process will take to acquire a single Scan. Measurements were taken at around 44, 96 and 144 weeks of age.
We applied a modified version of the clinically relevant FI38, which was calculated as the average of 31 traits that are indicators of age-associated deficits and health deterioration. Each trait was scored on a scale of 0, 0.5 or 1, where 0 indicated the absence of the deficit; 0.5 indicated mild deficit; and 1 indicated severe deficit. At the beginning of the 5 months, readings were taken, and repeated every 6 months. Simple averaging yielded a raw FI score of between 0 and 1 for each mouse. Frailty scores were adjusted by taking the random effects of coat colour and experimenter effects into account.
Over 100,000 values were collected for the 937 mice over the course of their lives. After local regression fitting within mouse, body weights were analysed.
We set up an independent cohort of 160 female DO mice to get an accurate estimate of food intake and changes in body weight when they fast on a weekly basis. Mice were placed on the same DR protocols as in the main study. Food was weighed daily as mice age from 30 to 36 and 43 weeks old. Daily and weekly averages were presented as daily and weekly data across timepoints by diet. The body composition was determined at 43 and 45 weeks with the use of a small 5-gauss magnet and a non-imaging nuclear magnetic resonance instrument. NMR data were used to detect changes in body weight and composition before and after fasting. Values of body weight, lean mass, fat mass and adiposity (100% × fat mass/total mass) pre-fasting were co-plotted with the difference between before and after fasting (Friday to Monday for AL and CR; Tuesday to Thursday for 1D IF; Tuesday to Friday for 2D IF).
DR was implemented by controlling when and how much food mice are given. Feeding schedules for DR were started at 6 months of age. All mice were fed a standard mouse chow diet (5K0G, LabDiet). The AL feeding group was provided with unlimited access to food and water. The mice were given unfettered access to food and water. Food was not given out for the next 24 to 48 hours for the 1D and 2D groups, on Wednesdays at 15. The amount of food and water allotted to CR mice was measured daily, and the mice were provided with unlimited access to water. Historical feeding data from DO mice gave us an estimates of the amounts based on the consumption of 3.43 g per mouse per day. For the 40% CR protocol, a gradual reduction in food intake was implemented: the mice were first subjected to 20% CR for 2 weeks, then to 30% CR for an additional 2 weeks, before transitioning to the full 40% CR. In the 2D IF protocol, mice were initially acclimatized to the 1D IF regimen for 2 weeks. Up to eight mice per pen were co-housed. When mice were placed in a co-housing cage, they were able to grab food directly from the bottom of the cage and not compete for it with one another. CR mice were provided with a 3 day ration of food on Friday afternoon, which resulted in weekly periods of feasting followed by a period of food deprivation of approximately 1 day for the 20% CR mice and 2 days for the 40% CR mice, comparable to the IF fasting periods. The 15:00 feeding time closely approximates the circadian alignment of feeding, starting just before the beginning of the dark cycle, which is the normal active and feeding time of day for mice. This timing has been shown to maximize lifespan extension in mice subjected to 30% CR8. The FRIDAY TRIPLE feeding can induce a feast–famine cycle, but there is no direct assessment of the health impacts.