Quantification of the number of neutrophils in the skin of WT, TgfbrN and wild-type mice by means of atomic force microscopy
The white blood cells, called neutrophils, form rings around areas where the skin has been penetrated. These rings trap pathogens, ensuring that they don’t penetrate into deeper tissues.
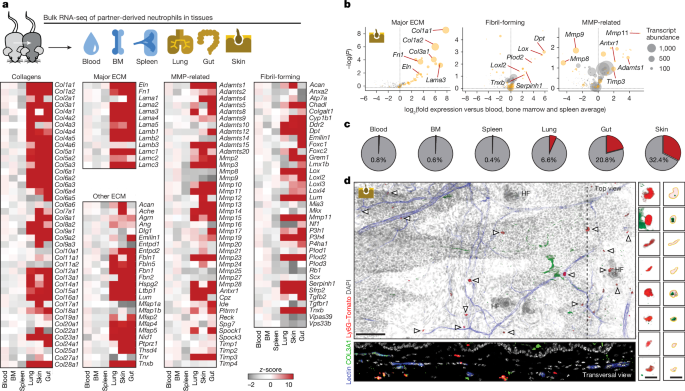
To investigate whether neutrophils have another trick up their sleeves, the researchers examined samples of mouse skin, lungs and gut ― organs that have contact with the external environment and are lined with protective layers to help ward off pathogens and foreign substances. Hidalgo and his colleagues found that a high percentage of neutrophils in these tissues produce collagen and other proteins that are important for forming ‘extracellular matrix’, a scaffolding that surrounds cells and gives tissues structure. The blood neutrophils didn’t releaseCollagen.
a, Representative images of neutrophils (green) stained for Col3a1 (red) in the skin of WT control (CreNEG Tgfbrflox littermates, 5 images from 2 mice) and TgfbrΔN mice (9 images from 3 mice), which we used to quantify the number of neutrophils per volume area is shown at right. Data are presented as mean values ± s.e.m., compared by two-tailed unpaired Student’s t-test. Histologic Characterization of the skin of wild-type control and TgfbrN mice, by hematoxylin-eosin staining, Masson’s trichrome for collagen-rich structures, and Ki The images are quantified in the graphs. The t-test was used to determine the P-values. c, Examples of SHG and fibre reconstruction using CT-FIRE for estimation of fibre width in control and TgfbrΔN mice also shown as distribution of widths in the histogram below (and in Extended Data Fig. Control and iDTR mice. P-values were determined by a two-sided unpaired Student’s t-tests. d, Schematics of the atomic force microscopy (AFM) set-up used to measure the stiffness of tissue samples (left) and its quantification in the form of elastic Young’s modulus in lung, intestine and skin of CreNEG;Tgfbrflox control (referred to here as WT; n = 5) and TgfbrΔN littermates (n = 5 mice). Each dot represents the median Young’s modulus value calculated from ∼250 individual force–distance analysed curves per mouse. Two-sided unpaired student was used to compare the data. There are height images and Young’s modulus maps for control and TgfbrN littermates. The ear skin was TEM images showing the skin and it’s parts, which showed the skin and its parts as well as the colour of the fibres. The subepidermal region has large collagen fibres in yellow circles. f, Quantification of fibre size in the skin of TgfbrΔN mice (19825 fibres) and CreNEG littermate controls (62279 fibres); data are from 2 independent experiments. The non-parametric test determined the P values. The mean values of the data are presented as mean values s.e.m., and the p- values are determined by a non-parametric test. h, Permeability assays in control (n = 7) and TgfbrΔN mice (n = 7) measured by FITC–dextran injected either intratracheally (for lung), or by oral gavage (for gut) in CreNEG control (n = 8) and TgfbrΔN mice (n = 8). Evans blue was given to control and TgfbrN mice and then measured. All controls here were Cre-negative Tgfbrflox littermates. Evans blue is being used in the ear skin of Mrp8Cre, as well as CreNEG and Tgfbr2flox mice. The numbers of replicates are displayed in the figure. Two-sided Student tests depletion methods against their controls or ANOVA with multiple comparison test to determine p-values. Box plots in (d,h–j) show median ± interquartile; whiskers show the range from minimum to maximum. The box plots in f show median interquartile; whiskers are defined by percentiles and the points outside this range are outliers.