Is it possible that better pain treatments are possible due to the presence of biomarkers?
Personalized Pain Medicine is Not For Everyone: Studying the Impact of Pain Treatment for Chronic Pain in the U.S. Military and Healthcare Networks
You don’t want to try the next drug until you know if it works. It’s possible that the drug causes side effects even if it eases the pain. After cycling through each migraine drug individually, the team started testing drug combinations. “We finally got control of her migraines on the 24th medication trial,” Russo says.
Personalized pain medicine doesn’t need to be perfect for it to offer a major improvement over today’s chronic-pain treatment. “Right now, as physicians,” Mackey says, “many times we’re doing a mental coin toss between possible treatments.” Even reaching an individual treatment prediction accuracy of 70–80% could reduce the frustration that a person feels and improve their quality of life, he says.
The US National Institutes of Health has also joined the hunt for pain metrics and wearable technologies. The agency has spent nearly $50 million on projects that aim to discover and assess a variety of pain conditions, including musculoskeletal pain, pain associated with the inheritance of the disorder sickle cell disease, and eye pain.
If a person breaks their arm mountain biking with friends on a weekend that they are going to celebrate a promotion, they won’t experience pain the same way as someone who breaks their arm cycling with their house in danger. Anxiety and depression can be risk factors for the onset of chronic pain. “Nobody experiencing chronic pain is in the same place at the same time,” she says. Everything that surrounds them, everything they experience, everything they are anticipating, and all the emotions involved in that experience are situated in a place of pain.
Deep learning can distinguish between people with chronic pain and patients with stimulator and stimulator based on basic neural network analysis: Application to a neuroscientists’ work with the IMI-PainCare consortium
It washed out to the human eye. A standard statistical analysis showed there was no difference when we compared pain sufferers with those who did not. A basic form of machine learning was tried to process the data, but the artificial intelligence algorithm could detect something. The program could differentiate between people with chronic lower back pain and people with a stimulator, as well as between people who wouldn’t use it and people who would. The technology shows that even a simple artificial Intelligence can beat conventional statistics for a task. “Basic machine learning is picking up signals that classic methods and the human eye are not capable of detecting.”
Vollert has seen similar results from applying basic machine learning to an approach called sensory phenotyping. He has used this technique with people experiencing neuropathic pain, which arises from nerve damage. The idea is to assess the broad changes to the sensory system that typically accompany pain. Many people get verysensitized to sounds and smells with a strong headaches.
The early results were promising. “In 2012, when I first published work using structural brain imaging to classify the presence or absence of lower back pain, we got 76% accuracy,” says Mackey5. There was progress but it ended in a stall. The accuracy hasn’t improved since then.
A better signal should emerge from pooling data from many different techniques. Vollert is currently involved in the IMI-PainCare BioPain consortium, led by Treede, a neuroscientist at the University ofHeidelberg, which aims to unite all the data sets. “To build really good models, we need prospective data sets that collect all kinds of marker information in parallel from the same patients,” Vollert says. That kind of data set is what we are generating right now.
With multimodal data in hand, advanced AI tools that are more powerful than the simple machine-learning models previously deployed will be crucial to categorizing people into subgroups and treatment selection, says Vollert. He states that they are working with machine-learning experts to find models that go deeper into the data.
Deep learning neural networks are a machine learning approach used for advanced artificial intelligence models. These models take an input data set and start to make connections between the data points, to generate their output. The goal is that combining deep learning neural networks with big new data sets will enable more fine-grained patient stratification and reach accuracies significantly higher than 80%.
Even given this opacity, physicians could still consider non-interpretable models’ output as one factor in their decision-making process for treatments, Mackey says. The next generation of artificial intelligence models might show their work more readily in the future. He says that research is moving towards models that are interpretable but which approach the accuracies of deep learning models.
Non-interpretable modelling presents a problem for pain-medicine practitioners, Mackey says. It is important for clinicians to understand the basis of a medical recommendation and not just rely on a machine’s word.
After coming under Russo’s care, it took three long years to find a way to control her migraines. The problem was not a shortage of options — there are plenty of treatments for people with chronic migraines, Russo says. Finding the right treatment for every chronic pain patient is very difficult to find and it takes a lot of trial and error.
She could have her ferocious segull with daily regularity. She would vomit and had to lie down during patient consults according to the pain doctor who treated her. “Then she’d have to get up, rinse her mouth and see the next patient.”
A user experience with a sensory-based platform for continuous objective pain assessment sensing systems (COMPASS) in the cold: Is it still worth trying?
“Hmm, it’s not really working,” says Lin, acknowledging that much optimization work remains. Maybe it’s time to stop. As I withdraw my hand from the icy cold, the pain quickly subsides — and I don’t need an objective measure to tell me when, after a few moments, the pain is gone.
The platform proved glitchy. With my hand submerged in a bucket of ice water, the objective pain scores displayed on the app generally trended upwards. The readings were inconsistent, however, fluctuating as I experienced a steadily increasing sensation of pain.
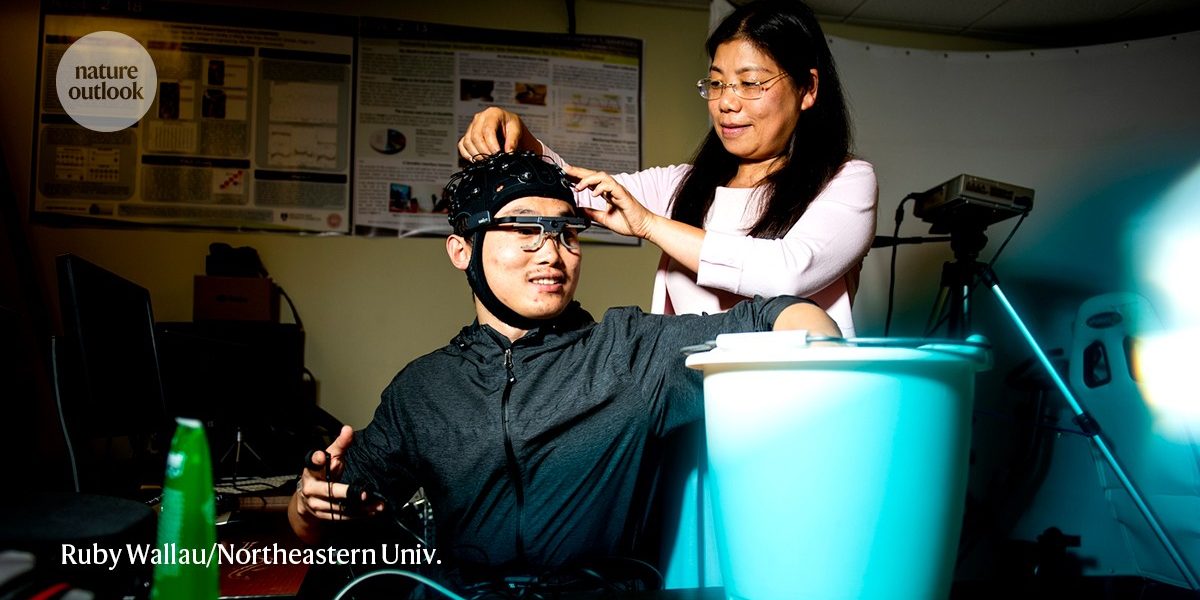
When I tried the cold-exposure task in her lab, I had only one sensor on my body feeding data into COMPASS. Monitoring of brain activity and measuring pupil diameter were not recorded.
A model based on facial expressions and brain activity signals that were only used for a subset of indicators was found to be better than other models when evaluated against self-reported pain scores. Front. Neurosci 16, 832617; 2022.
Lin has been fine-tuning her platform called Continuous Objective Multimodal Pain Assessment Sensing System (COMPASS) using data from people with chronic low back pain to validate her group’s machine-learning algorithms. The artificial-intelligence tools were able to pull insights from diverse data sets that align closely with peoples own pain assessments.
AlgometRx’s technology provides a snapshot of a person’s pain at a point in time. That can be fine for some situations, such as an assessment of a physician, but it can not track fluctuations in pain levels over time.
But the cost and complexity of brain-recording technologies make these methods unsuitable for clinical use. “It has to be practical and portable,” Saab says — which is where simpler diagnostic tools and wearable devices come in.
The platform is only allowed in the United States for use with people under anaesthesia and unable to communicate their pain levels. By allowing clinicians to adjust painkiller dosages in line with an individual’s pain, it aims to optimize pain management and mitigate the risks associated with unnecessary opioid prescriptions.
Moreover, many individuals — including babies, people who are non-verbal, people who are critically injured and those under sedation — cannot communicate their symptoms effectively. It complicates the management of pain and the delivery of analgesia.
As discomfort escalates to pain, a sensor strapped to my chest detects changes in my heart rate, breathing pattern, skin conductance and other bodily responses. There are signals from the body that are processed to create a pain score. Displayed on a smartphone app, my pain level is 4.
The graduate student bears down on my arm with a force akin to a firm handshake. The sensation of pressure on a patch of skin smaller than a small coin starts to hurt when it’s concentrated on.